May Puzzling Phenomenon: Fire and Ice
Concept
Phase changes/energy transfers, with possible Earth science connections to volcanic eruptions and the rock cycle
Introduction & Phenomenon
What happens when molten rock meets ice? Or, more precisely, when rock is heated to such an extreme temperature that it is a flowing liquid, like in an erupting volcano, what happens when that superheated rock flows out onto a sheet of ice?
https://www.youtube.com/embed/yvSmPqqZB3Q (Watch on mute, as some of the scientific explanation is provided in the audio.)
Explanation of science involved
A basic knowledge of the world and how heat and substances behave would lead most students to predict that the molten rock would instantly melt the ice, maybe boring a deep hole in the ice or creating a rush of water. But what happens instead is that the fiery liquid flows over the solid ice, bubbling and steaming, and the rock gradually cools and solidifies in a billowy shape.
So instead of observing a phase change from solid to liquid, we observe a phase change from solid directly to gas. The technical word for this is sublimation, and it occurs when the temperature and pressure conditions are both below the triple point in a substance’s phase diagram. In simple terms, the thermal energy in the molten rock is so much greater than that in the ice that when the magma touches the ice it causes the ice molecules to instantly move so fast that they skip their liquid phase and go directly to water vapor. This helps also to explain the shape of the cooled magma—it looks almost like a bunch of bubblegum bubbles because the released water vapor gets trapped inside the molten rock as it cools.
Further Ideas for Classroom Use
This puzzling phenomenon could kick off a lesson on phase changes within a unit on interactions of matter, or even a lesson on energy transfers because it illustrates the relationship between thermal energy and kinetic energy of particles. Some key questions for discussion might include:
- Why doesn’t the ice melt into water when the molten rock touches it?
- Where are the bubbles coming from?
- What is happening at the molecular level in the ice?
- How is energy transferred between the lava and ice?
Students could draw an explanatory model of what they think is happening to cause the observed phenomenon; depending on the curricular context, models could focus on energy transfers with arrows, etc., and/or on the movement of molecules in the two substances before and after they come into contact with one another.
A phase diagram for water could make the conversation more quantitative, incorporate graph analysis, and provide a model for students to analyze other phase diagrams in groups and individually:
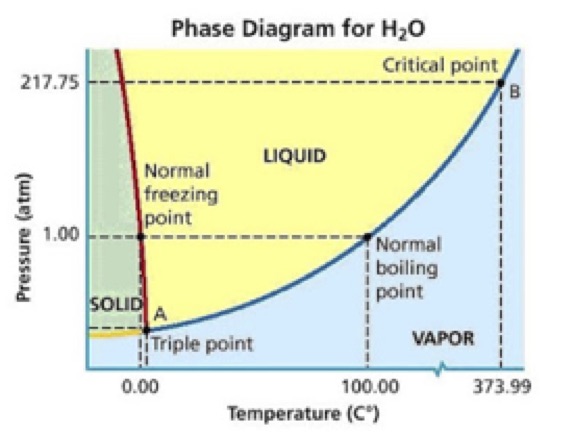
There are obvious connections to Earth science as well, asking students to place this video event on the rock cycle, and conjecture about what type of rock is formed when this lava cools based on its appearance. Students could also conjecture about where on the globe this phenomenon might occur in the real world—where are there active volcanoes in glacial environments? How might volcanic eruptions differ in a cold environment versus one in a tropical environment? What might we expect to observe if molten rock came to Earth’s surface underwater and why (bubbles forming from the created water vapor and rising to the surface)?
Download this month's Scientific Phenomenon as a Word Document here.